SAFETY SUMMARY WITH WARNINGS
WARNINGS - Olumiant may cause serious side effects, including:
- Serious infections, including tuberculosis (TB), shingles, and others caused by bacteria, fungi, or viruses. Some people have died from these infections. Olumiant can make you more likely to get infections or make any infections that you have worse. Your doctor should test for TB before starting Olumiant and watch for TB symptoms during treatment. You should not start Olumiant if you have any kind of infection unless your doctor tells you it is okay. While taking Olumiant, tell your doctor right away if you have symptoms of an infection, such as: If you get a serious infection, your doctor may stop Olumiant until your infection is controlled.
- fever, sweating, or chills
- muscle aches
- cough
- shortness of breath
- blood in phlegm
- weight loss
- warm, red, or painful skin or sores on your body
- diarrhea or stomach pain
- burning with urination or urinating more often than normal
- feeling tired
- Increased risk of death in people 50 years of age or older who have at least 1 heart disease risk factor and are taking a medicine in a class of medicines called JAK inhibitors.
- Cancer and immune system problems. Olumiant may increase your risk of lymphoma and other cancers, including skin cancers. People taking a medicine in the class of medicines called JAK inhibitors have a higher risk of certain cancers, including lymphoma and lung cancer, especially if you are a current or past smoker. Follow your doctor’s advice about having your skin checked for skin cancer while taking Olumiant.
- Increased risk of major cardiovascular events such as heart attack, stroke or death in people 50 years of age and older who have at least 1 heart disease risk factor and taking a medicine in the class of medicines called JAK inhibitors, especially if you are a current or past smoker. Get emergency help right away if you have any symptoms of a heart attack or stroke while taking Olumiant, including:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
- weakness in one part or on one side of your body
- slurred speech
- Blood clots in the veins of your legs or lungs, and arteries. This may be life-threatening and cause death. Blood clots in the veins of legs and lungs have happened more often in people who are 50 years of age or older and with at least 1 heart disease risk factor taking a medicine in the class of medicines called JAK inhibitors. Stop taking Olumiant and tell your doctor or get emergency help right away if you have any signs and symptoms of blood clots, including swelling, pain or tenderness in the leg, sudden chest pain, or shortness of breath, while taking Olumiant.
- Allergic reactions. While taking Olumiant, if you have symptoms, such as rash (hives), trouble breathing, feeling faint or dizzy, or swelling of your lips, tongue, or throat, stop taking Olumiant and get emergency help right away. Some of these reactions seen in people taking Olumiant were serious.
- Tears in the stomach or intestines. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or methotrexate. While taking Olumiant, tell your doctor right away if you have fever and stomach-area pain that does not go away, and a change in bowel habits.
- Changes in laboratory test results. Your doctor should do blood tests before and while taking Olumiant. You should not take Olumiant if your white or red blood cell count is too low or your liver tests are too high. Your doctor may pause your treatment with Olumiant because of changes in these test results. Your doctor should also check your cholesterol levels approximately 12 weeks after you start Olumiant and as needed.
Common side effects
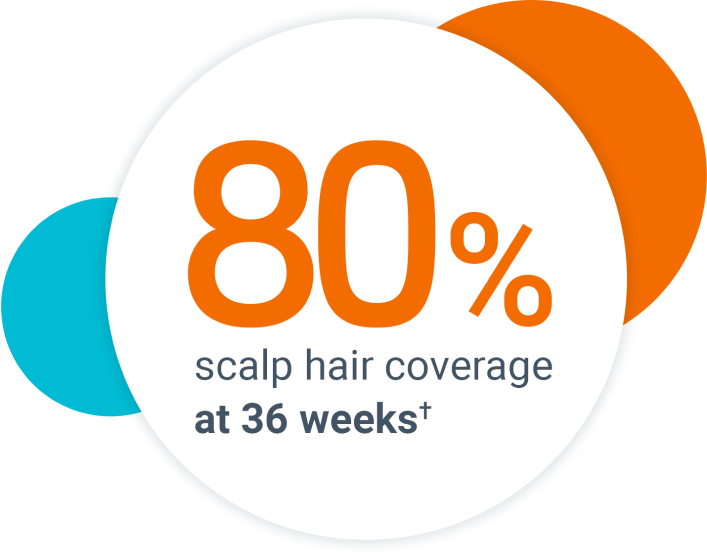
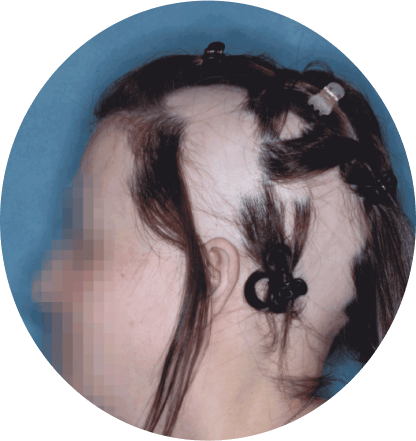
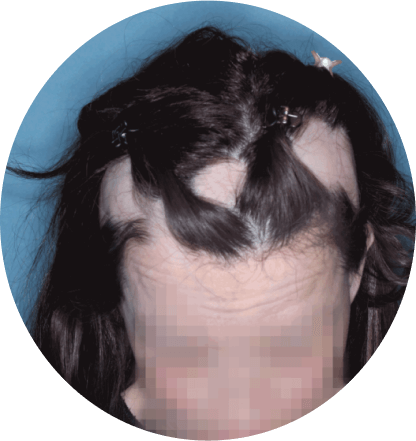
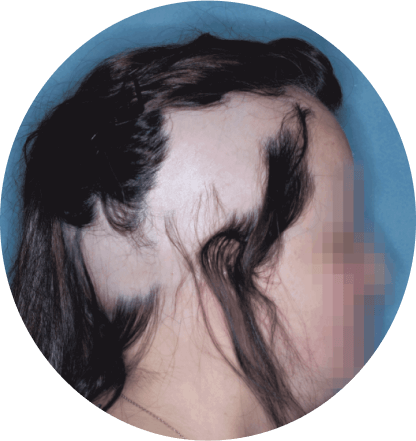
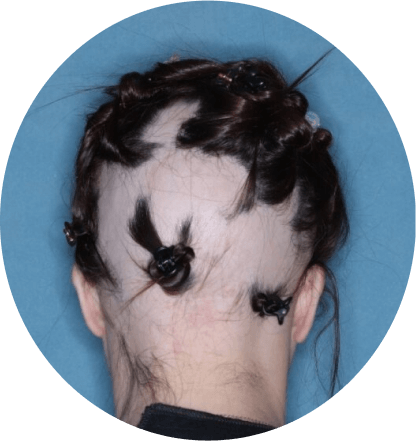
The most common side effects of Olumiant in people treated for alopecia areata include:
- upper respiratory tract infections (cold or sinus infections)
- headache
- acne
- increased cholesterol levels
- increased muscle enzyme levels
- urinary tract infection
- increased liver enzyme levels
- inflammation of hair follicles (folliculitis)
- tiredness
- lower respiratory tract infections
- nausea
- genital yeast infection
- low red blood cell count (anemia)
- low white blood cell count (neutropenia)
- stomach-area (abdominal) pain
- shingles (herpes zoster)
- increased weight
The most common side effects of Olumiant in people treated for rheumatoid arthritis include:
- upper respiratory tract infections (cold or sinus infections)
- nausea
- herpes simplex virus infections, including cold sores
- shingles (herpes zoster)
These are not all the possible side effects of Olumiant. Tell your doctor if you have any side effects. You can report side effects to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Before using
Before you use Olumiant, tell your doctor if you:
- Are being treated for an infection, have an infection that won’t go away or keeps coming back, or think you have symptoms of an infection.
- Have TB or have been in close contact with someone with TB.
- Have had shingles (herpes zoster).
- Have had hepatitis B or C, cancer, or blood clots in the veins of your legs or lungs.
- Live, have lived, or have visited parts of the country that increase your risk of fungal infections. These may include the Ohio and Mississippi River valleys and the Southwest. Ask your doctor if you do not know if you have lived in an area where these infections are common.
- Are a current or past smoker.
- Have had a heart attack, other heart problems or stroke.
- Have other medical conditions, including kidney or liver problems, low blood cell counts, diabetes, lung disease, HIV, or a weak immune system.
- Have any stomach-area pain or have been diagnosed with inflammation in the large intestine (diverticulitis) or ulcers in your stomach or intestines.
- Have recently received or plan to receive a vaccine. People taking Olumiant should not receive live vaccines.
- Are pregnant or plan to become pregnant. It is not known if Olumiant may harm your unborn baby. If you become pregnant while taking Olumiant, call Eli Lilly and Company at 1-800-545-5979 to report the pregnancy.
- Are breastfeeding or plan to breastfeed. You should not breastfeed while taking Olumiant and for 4 days after the last dose. Talk to your doctor about the best way to feed your baby while taking Olumiant.
- Are taking other medicines, including prescription and over-the-counter medicines, vitamins, and herbal supplements. It is especially important to tell your doctor, if you take:
- a medicine called probenecid
- medicines that affect your immune system, such biologic medications, other JAK inhibitors, or strong immunosuppressants (such as azathioprine or cyclosporine) since these may increase your risk of infection.
- Are under age 18. It is not known if Olumiant is safe and effective in children.
How to take
- Take Olumiant exactly as your doctor says.
- Take Olumiant once a day by mouth with or without food.
- Talk to your doctor if you cannot swallow tablets whole.
- If you take too much Olumiant, call your doctor or poison control center at 1-800-222-1222, or go to the nearest hospital emergency room right away.
Learn more
Olumiant is a prescription medicine. For more information, call 1-800-545-5979.
This summary provides basic information about Olumiant but does not include all information known about this medicine. Read the information that comes with your prescription each time your prescription is filled. This information does not take the place of talking with your doctor. Be sure to talk to your doctor or other healthcare provider about Olumiant and how to take it. Your doctor is the best person to help you decide if Olumiant is right for you.
BA CON BS 14SEP2022
Please see full Prescribing Information, including Boxed Warning, and Medication Guide.